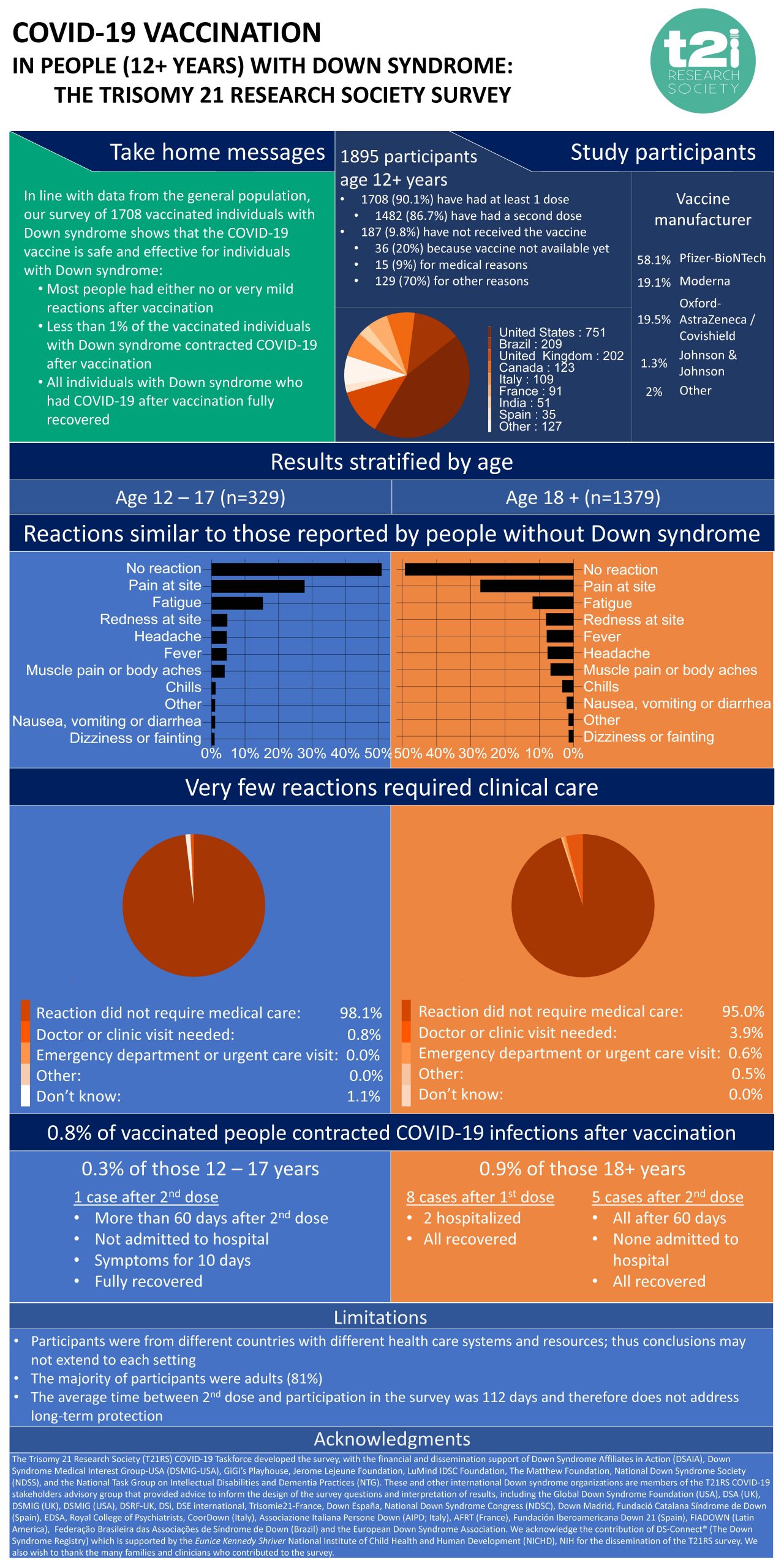
Research from the Trisomy 21 Research Society (T21RS) has shown that Covid-19 vaccinations are safe to use in people who have Down’s syndrome aged 12 and above.
The survey collected information from nearly 2,000 individuals who have Down’s syndrome to determine their response to vaccination. Results showed that the majority experienced either no reaction or only mild symptoms such as pain or redness at the injection site, or tiredness. These are common reactions which also occur in the general population.
In those who experienced symptoms due to vaccination, the vast majority (>95%) resolved by itself and did not require medical care. The vaccines appeared to be safe to use in both young people (12 – 17 years) and adults (18 and older) who have Down’s syndrome.
The survey also showed that the vaccines help to protect people with Down syndrome’s from developing severe Covid-19 illness. A small number (14 people; 0.8%) contracted Covid-19 after vaccination and more than half of these infections occurred between the first and second dose of the vaccine (eight people), so before they were considered fully vaccinated. Of the 14, only two individuals (who both had only one vaccination) were admitted to hospital. All of those who contracted Covid-19 after either full or partial vaccination recovered.
It has been well established that people who have Down’s syndrome have more severe disease when they are infected with SARS-CoV-2, the virus that causes Covid-19. Such studies have estimated that individuals with Down’s syndrome have a five-fold higher risk for hospitalisation, and between three to 10-fold higher risk for death when they develop Covid-19, with a higher risk of death among elderly individuals who have Down’s syndrome.
In many countries, having Down’s syndrome has been designated as a priority condition for vaccination. This new data show that the vaccine appears to be safe and effective in people who have Down’s syndrome, and provide evidence that vaccination will help protect them from becoming severely ill with Covid-19.
The Trisomy 21 Research Society says it strongly supports vaccination prioritization and booster vaccination of people who have Down’s syndrome.
Analysis of various data sources collected during the Covid-19 pandemic has established that individuals with Down syndrome are at increased risk for both hospitalisation and mortality after infection with SARS-CoV-2. Adults with Down syndrome were amongst the highest risk groups for mortality. Furthermore, there have been concerns that, when admitted to hospital during peak infection periods and when demands on resources are high, people with disabilities and long-term conditions may not be prioritised for access to scarce resources such as respiratory ventilation or intensive care beds.
It has been the clinical experience of many Down syndrome clinics in different countries that Covid-19 vaccines can be safely given to children (from age 12) and adults with Down syndrome. There are also reasonable indications that full vaccination offers significant protection against the poor outcomes associated with infection in people with Down syndrome. However, based on our understanding of the immune response in Down syndrome, including reduced numbers of and differences in memory B cell responses, and previously published studies on the immunological response of those with Down syndrome to other vaccines, their antibody response to vaccination may be less robust when compared to peers without Down syndrome.
We therefore recommend that individuals with Down syndrome should be amongst those prioritised for booster vaccination for persistent production of antibodies against Covid-19 antigen. There have been some indications of additional benefit by combining vaccines from different sources (e.g. Pfizer and Moderna, or Moderna and AstraZeneca) to promote better immune responses based upon studies in the general population, but any booster is preferable to none. (Trisomy 21 Research Society statement)
The DSA and many other international Down’s syndrome organizations are members of the T21RS Covid-19 stakeholders advisory group.